Engaged in medical equipment production, the following conditions should be provided:
(1) There is a production site, environmental conditions, production equipment, and professional technicians that have been adapted to the production of medical instruments;
(2) A mechanism or full-time inspectorian and inspection equipment for quality inspection of medical equipment production;
(3) There is a management system that guarantees the quality of medical equipment;
(4) There is a after-sales service capability that is adapted to the medical device of the production;
(5) Compliance with product development, production process documents Domestic secondary, three types of medical equipment registration flow chart:
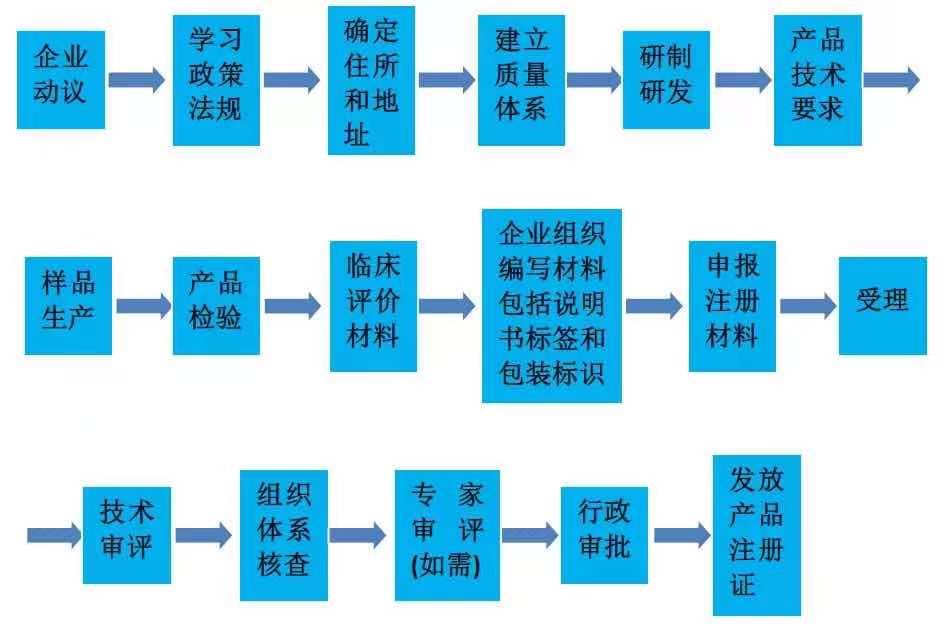
Apply for production license If you open a second class, the third type of medical device production enterprise shall apply to the production of production licenses to the city, autonomous region, and municipalities directly under the Central Government, and submit the following information:
(1) A copy of the business license, organizational code certificate;
(2) Copy of the registration certificate and product technical requirements for the production of medical devices held by the enterprise;
(3) A copy of the identity certificate of legal representative and corporate responsible person;
(4) Copy of the identity, academic qualifications, and title certificate of production, quality and technical person in charge;
(5) Production management, quality inspection position, professional qualifications, title list;
(6) Certification documents of production site, there is a special production environment requirement to submit a copy of the facility and environment;
(7) Main production equipment and inspection equipment catalogs;
(8) Quality manual and program document;
(9) Process flow chart;
(10) Officer authorization certificate;
(11) Other proof information.

